MGN-3/Biobran is a denatured hemicellulose obtained by reacting
rice bran hemicellulose with multiple carbohydrate hydrolyzing enzymes from
Shiitake mushrooms. Over the last 24 years, our fundamental research objective
has been to study the biotherapeutic activity of MGN-3 as a treatment for
cancer based on its ability to activate the immune system. This objective has
been pursued in vitro,
and in animal and human studies. This review is focused on the immunomodulatory
effects of MGN-3 and on its potential as an anticancer agent. In vitro studies
showed that culturing different human and murine cancer cell lines with MGN-3
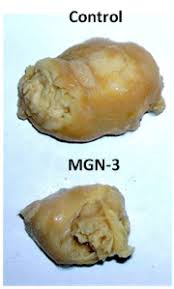
resulted in a reduction of the survival rate of cancer cells. In vivo studies
have also shown that MGN-3 induces tumor regression in several models of animal
bearing tumor, including gastric cancer, neuroblastoma, and Ehrlich carcinoma.
In addition, the anti-cancer activity of MGN-3 has been shown in human clinical
trials and in several case reports on patients with Hepatocellular Carcinoma
(HCC) and progressive and partially metastasized cancer. Patients that were
treated with MGN-3 in addition to Conventional Therapy (CT), as compared with
CT alone, showed: 1) less recurrence of cancer, 2) higher survival rate and 3)
improved Quality of Life (QOL) as characterized by improvements in physical
activity, appetite, sleep, and digestion, and a decrease in pain and anxiety.
This review summarizes the preclinical and clinical research on
MGN-3/ Biobran since it was first patented in 1992. Various animal studies and
human clinical trials including different types of malignancies have
demonstrated that MGN-3 is a potent Biological Response Modifier (BRM). MGN-3
enhances the cytotoxic reactivity of immune cells with anti-cancer activity
such as NK and CD8+ T cells via increasing cell granularity, stimulates the
production of interferons, IL-2 and IL-12, and functions as a natural adjuvant
for Dendritic Cells (DC). Therefore, MGN-3 may be used in DC-based vaccine
strategies against infections and cancer. Importantly, MGN-3 is a unique BRM
because it is a safe non-toxic agent and does not exhibit hyporesponsiveness.
MGN- 3 has the potential to be a novel and promising immune modulatory adjuvant
that could complement the existing immunotherapeutic modalities for cancer
patients.
Despite the last decade of advances in treatment options, cancer
remains the second leading cause of death in the United States. Unfortunately
the outcome of standard cancer treatments is often poor due to the emergence of
Multidrug Resistance (MDR) during the course of treatment. MDR cells are a
significant factor in the failure of chemotherapeutics as evidenced by high
relapse rates for the majority of patients. Therefore, to increase cancer
survival and improve symptom control, there is a strong need for new and better
approaches to cancer treatment. Today, the National Cancer Institute (NCI) has
acknowledged the importance of immune therapy for the treatment of cancer. NCI,
other health organizations, and professionals in the field of oncology are
currently working to harness the immune system to fight cancer and to expand
immunotherapy in combination with other types of cancer treatment, such as
targeted therapy, chemotherapy, and radiation therapy.


No comments:
Post a Comment